-
-
-
- Assistenza per bioprocessi
- Assistenza per centrifughe e rotori
- Assistenza per Mastercycler
- Assistenza per l'automazione
- Assistenza per ultracongelatori
- Assistenza per incubatori
- Assistenza per agitatori
- Assistenza per la fotometria
- Assistenza per regolazione termica e miscelazione
- Assistenza per pipette
-
-
-
-
- Assistenza per bioprocessi
- Assistenza per centrifughe e rotori
- Assistenza per Mastercycler
- Assistenza per l'automazione
- Assistenza per ultracongelatori
- Assistenza per incubatori
- Assistenza per agitatori
- Assistenza per la fotometria
- Assistenza per regolazione termica e miscelazione
- Assistenza per pipette
-
-
- Centrifughe da banco
- Centrifughe da pavimento
- Centrifughe refrigerate
- Microcentrifughe
- Centrifughe multiuso
- Centrifughe ad alta velocità
- Ultracentrifughe
- Concentratore
- Prodotti IVD
- High-Speed and Ultracentrifuge Consumables
- Provette per centrifughe
- Piastre per centrifughe
- Gestione degli apparecchi
- Gestione di campioni e informazioni

Handling of Hazardous Material
Lab Academy
- Microbiologia
- Sicurezza e biosicurezza
- Centrifughe e rotori
- Saggio
Working in clinical diagnostic laboratories usually means working with potentially infectious samples like blood or other bodily fluids. But handling infectious microorganisms or harmful chemicals is quite common in research laboratories as well. To ensure the safety of laboratory personnel and to prevent laboratory acquired infections (LAIs) or other health hazards, reasonable precautions must be taken throughout the whole workflow.
Instead of examining the entire flow of work now, we will concentrate on the centrifugation step of infectious and hazardous samples as the dangers here are often quite underestimated. Statistics show that about 80% of all LAIs are unsuspected (aerosols). For further information, please see the additional literature listed at the end of this text.

Routes of laboratory acquired infections
Individuals who work with potentially infectious samples face many risks in both clinical diagnostic laboratories and in research laboratories. Because exposure to harmful or infectious substances occurs more often than expected, raising awareness about the risks and sources of LAIs as well as about the safety precautions lab workers need to take is very important. Certainly not every exposure leads to an infection, but the less the exposure, the lower the risk for acquiring an LAI.
The most significant routes of laboratory acquired infections are:
- Spills and splashes on skin and mucous membranes as well as contact with spills and droplets on surfaces (dermal contact)
- Ingestion through mouth pipetting or touching the mouth (or eyes) with fingers or contaminated work equipment
- Inhalation of infectious aerosols
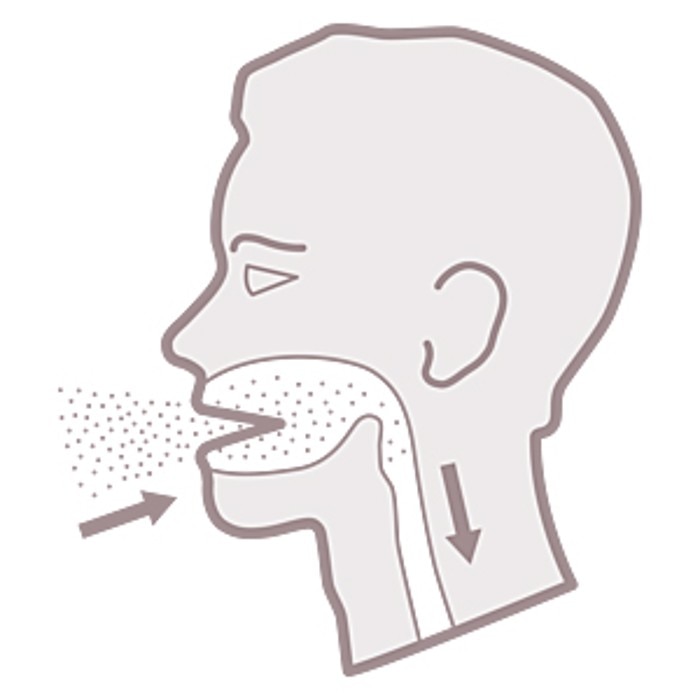
Centrifuges as one source of aerosols
Due to their mode of operation, centrifuges can present a high risk if not operated correctly. To maintain a desired temperature, air-cooled and refrigerated centrifuges use an air ventilation system. This system discharges warm air from inside the centrifuge into the environment.
Infectious agents present in the exhaust air will be dispersed quickly and broadly throughout the laboratory. Tube breakage poses an even greater risk, since this can produce a large amount of aerosol. The use of aerosol-tight lids or caps will reduce this risk significantly.
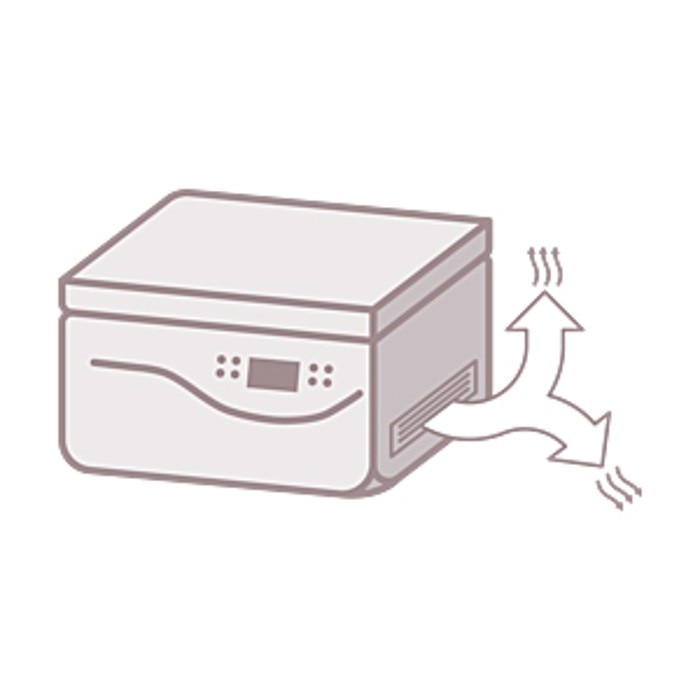
Taking precautions
To ensure lab workers are optimally protected, a couple of safety guidelines should be followed.
First, and very important, is factoring in human error:
- Young, inexperienced workers are more likely to make errors; therefore, regular training and supervision are necessary.
- A lack of mental alertness, high stress levels, and heavy workloads are factors that can lead to less careful behavior.
- Suitable equipment must be provided and properly maintained; this includes both technical equipment and protective gear.
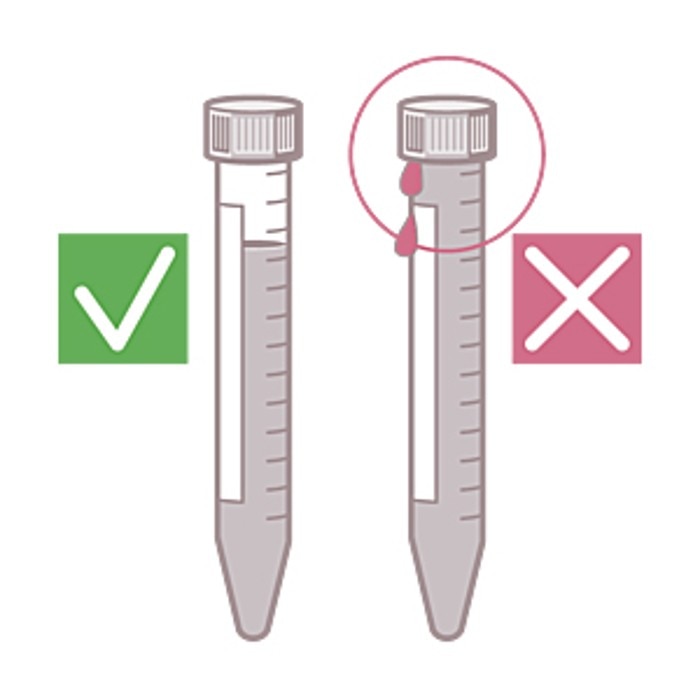
For aerosol-tight centrifugation, both aerosol-tight caps or lids and suitable vessels must be used. In this regard, the following points need to be considered:
- Material. Use nonbreakable materials such as PP, PE, and the like; avoid glass tubes.
- Leak tightness. Make sure the vessel can be closed and remain leak-tight; use, for example, Eppendorf tubes.
- Filling volume. Every vessel has a maximum filling volume, usually two-thirds or 80% of the total volume (called the “working volume”) that shouldn’t be exceeded; this ensures the liquid in the vessel does not touch the vessel lid.
- Droplets. These can occur in the thread of the vessel lid or even on the outside of the vessel. These droplets can become airborne during centrifugation and thereby form aerosols.
- Loading and unloading. Aerosol-tight caps and lids do not prevent formation of aerosols during centrifugation; rather, they ensure aerosols cannot leak from the closed system. Therefore, you must wait at least 10 minutes before opening the bucket or rotor. This gives the aerosols time to settle down. Also, you should load and unload the buckets or rotor in a biosafety cabinet (especially in virology and mycobacteriology) to minimalize the risk of escaping aerosols.
- Disinfection and autoclaving. Buckets, caps, rotors, and lids are the main components that come into contact with aerosols. Correctly handling them does prevent aerosols from being dispersed within the rest of the device, but the parts themselves do need to be disinfected after each use. This is done using suitable disinfectants and following to the manufacturer’s recommendations (usually 70% ethanol works fine for rotors and buckets). You can also regularly autoclave rotors, rotor lids, buckets, and caps (please refer to the operating manual for parameters; autoclaving is usually carried out for 20 minutes at 121°C, 2 bar).
- Seals. All aerosol-tight caps and lids are delivered with rubber seals. Together, the caps and seals form an aerosol-tight unit, which must be tested and certified by an independent test institute (e.g., Public Health England, Porton Down, UK). You need to regularly check the seals to ensure they are intact, nonporous, and correctly seated in the grooves. If any of these factors are not present, aerosol-tightness cannot be guaranteed. If necessary, grease or even replace the rubber seal.
Using centrifuges in a safe way
The centrifuge itself is another factor to be considered in the safety workflow:- Speed limits. Exceeding the speed limits of rotors can lead to tube breakage or even a rotor crash, therefore the rotor speed limit must be considered when the centrifuge is not equipped with automatic rotor recognition (a feature that ensures the maximum speed is not exceeded).
- Tube breakage. Should a tube break or leak, do not open the centrifuge for at least 30 minutes after the run. Since this cannot always be detected before you open the buckets or rotor (a sudden imbalance can be a first sign of tube breakage), we recommend waiting at least 10 minutes at all times before you open the containers.
- Maintenance. Good maintenance and regular checks of the centrifuge, rotors, and equipment is necessary for ensuring safety and preventing system failure (e.g., rotor crash, which leads to a large amount of aerosol escaping with the centrifuge exhaust). Please refer to the manufacturer’s instructions for maintenance information or watch our centrifuge maintenance video.
Personal protective gear is another crucial factor in avoiding contact with infectious or harmful substances (mostly through ingestion and dermal contact) and the following should be heeded:
- Protective gear. Always wear a laboratory coat, safety gloves, and safety goggles when working with infectious or hazardous substances. This will minimize the risk of dermal or mucous contact with the material (splatter, droplets, etc.)
- Clean hands. After working with infectious material, remove the used gloves and disinfect your hands before washing them thoroughly.
- Clean gear. Be sure personnel protective gear, including laboratory coats, safety goggles, and the like, are regularly cleaned; replace if damaged.
Including these precautions in the workflow will provide a higher level of security during centrifugation as well as minimize the risk of LAIs.
References:
https://www.cdc.gov/Mmwr/preview/mmwrhtml/su6101a1.htm
(Guidelines for Safe work Practices in Human and Animal Medical Diagnostic Laboratories)
Book: Collins & Lyne's Microbiological Methods (8th Edition, Arnold, 2004, Chapter 1, Safety in microbiology)
Meno informazioni
